Days after Texas Governor Greg Abbott said that he was receiving the monoclonal antibody infusion therapy as a treatment for COVID-19, it seems the treatment has become more popular in the Fort Worth area.
Just recently, Fort Worth opened the new Regional Infusion Center, one of six centers run by the Texas Department of State Health Services. It has the capacity to treat 90 patients daily, with each procedure requiring an IV and lasting for about an hour. According to Evy Ramos, a spokesperson for BCFS Health and Humans Services Emergency Management Division, the patient is also made to wait at the center for one more hour after the treatment to make sure they don’t show any adverse reactions.
The center had treated 40 people through Monday, according to Douglas Loveday, a spokesperson for the Texas Department of State Health Services.
In addition to the new infusion centers, other hospitals and clinics managed by the Texas Division of Emergency Management also provide the treatment.

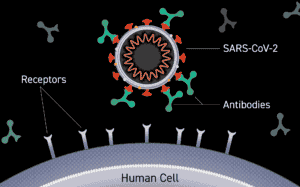
In terms of the treatment process, Meenakshi Ramanathan, an infectious diseases clinical pharmacist in Tarrant County says that the new Regional Infusion Center uses Regeneron Pharmaceuticals’ treatment process. It is a two-fold treatment that uses two types of monoclonal antibodies: casirivimab and imdevimab, to decrease the body’s viral load or the amount of virus in a person’s body by infusing with natural antibodies and neutralizing foreign objects in the system. The monoclonal infusion therapy also slows hospitalizations as it works by speeding up the body’s defenses against threats.
Ramanathan likened the monoclonal antibody infusion treatment process to cultivating vegetables in one’s own garden, which is faster than even having them delivered from elsewhere.
The infusion therapy is meant to treat people with a mild to moderate case of COVID-19, long with people who have been exposed to the virus. It was first authorized by the FDA for emergency use in November 2020, though they clarified that it is not a substitute for the COVID vaccines.
Ramanathan also said that the popularity of the treatment seems to be rising, as even Texas Gov. Abbott and former President Donald Trump have both received Regeneron’s treatment. He also said that the treatment works against the four main variants of coronavirus in the U.S., including the highly transmissible Delta variant.